荧光淬灭

荧光淬灭(英语:Quenching)是指荧光物质的荧光强度降低的任何过程。许多过程都会导致淬灭现象,例如激发态反应、能量转移、配合物的形成和碰撞淬灭。氧分子、碘离子和丙烯酰胺都是常见的化学淬灭剂[1],氯离子是常见的奎宁淬灭剂[2][3][4]。
淬灭被用于制造光极传感器,例如氧对某些钌配合物的淬灭效应可以用于测量溶液中的氧饱和度;淬灭是荧光共振能量转移(FRET)分析技术的基础[5][6][7];特定标靶分子结合时的淬灭与去淬灭效应可用作光学造影剂 [8][9];许多染料有自淬灭现象,导致荧光显微镜的蛋白质-染料偶联物的亮度降低[10];或是用作检测蛋白酶解传感器[11]。
淬灭机理
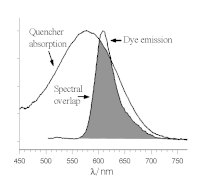
荧光共振能量转移
|
主条目:荧光共振能量转移 |
荧光淬灭可以用多种机制解释,其中的FRET是动态淬灭机制,供体与受体通过非辐射的方式转移能量。因为能量转移的过程基于二者的跃迁偶极间的经典偶极-偶极相互作用,因此极其依赖于供体-受体间的距离R,以E∝1/R6的效率下降[12],通常FRET过程在100 Å的距离内发生。FRET的过程还取决于供体-受体的重叠(如图)以及跃迁偶极矩的相对取向。
德克斯特电子转移
|
主条目:德克斯特电子转移 |
德克斯特电子转移(或称为德克斯特电子交换、碰撞能量转移等)是另一种动态淬灭机制[13],同样此过程依赖于供体和受体间的距离,能量转移速率由下式所示,此过程通常在10 Å的距离内发生[14],式中是供体与受体间的距离,与是不易由实验得到的常量,是重叠光谱的积分[15]。
激基配合物
|
主条目:激基缔合物 |
激发态配合物的形成过程是第三种动态淬灭过程。

静态淬灭
剩余的能量转移机理是静态淬灭,静态淬灭发生在基态的荧光分子与淬灭剂通过结合形成配合物时,即激发发生之前。静态淬灭形成的配合物会失去荧光性。
碰撞淬灭
激发态的荧光基团与淬灭剂原子或分子碰撞时,以无辐射跃迁的方式回到基态,此种淬灭过程称为碰撞淬灭。
参考文献
- ^ Acrylamide and iodide fluorescence quenching as a structural probe of tryptophan microenvironment in bovine lens crystallins. Phillips SR, Wilson LJ, Borkman RF. Curr Eye Res. 1986 Aug;5(8):611-9.
- ^ Fluorescence experiments with quinine James E. O'Reilly J. Chem. Educ., 1975, 52 (9), p 610 doi:10.1021/ed052p610
- ^ Photophysics in a disco: Luminescence quenching of quinine LouAnn Sacksteder , R. M. Ballew , Elizabeth A. Brown , J. N. Demas , D. Nesselrodt and B. A. DeGraff J. Chem. Educ., 1990, 67 (12), p 1065 doi:10.1021/ed067p1065
- ^ Halide (Cl-) Quenching of Quinine Sulfate Fluorescence: A Time-Resolved Fluorescence Experiment for Physical Chemistry Jonathan H. Gutow J. Chem. Educ., 2005, 82 (2), p 302 doi:10.1021/ed082p302
- ^ Peng X, Draney DR, Volcheck WM. Quenched near-infrared fluorescent peptide substrate for HIV-1 protease assay. Achilefu S, Bornhop DJ, Raghavachari R (编). Optical Molecular Probes for Biomedical Applications 6097. 2006: 60970F. S2CID 98507102. doi:10.1117/12.669174.
- ^ Peng X, Chen H, Draney DR, Volcheck W, Schutz-Geschwender A, Olive DM. A nonfluorescent, broad-range quencher dye for Förster resonance energy transfer assays. Analytical Biochemistry. May 2009, 388 (2): 220–8. PMID 19248753. doi:10.1016/j.ab.2009.02.024.
- ^ Osterman H. The Next Step in Near Infrared Fluorescence: IRDye QC-1 Dark Quencher.. Review Article. 2009, 388: 1–8. (原始内容存档于20 March 2020).
- ^ Blum G, Weimer RM, Edgington LE, Adams W, Bogyo M. Comparative assessment of substrates and activity based probes as tools for non-invasive optical imaging of cysteine protease activity. PLOS ONE. July 2009, 4 (7): e6374. Bibcode:2009PLoSO...4.6374B. PMC 2712068
 . PMID 19636372. doi:10.1371/journal.pone.0006374
. PMID 19636372. doi:10.1371/journal.pone.0006374  .
.
- ^ Weissleder R, Tung CH, Mahmood U, Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nature Biotechnology. April 1999, 17 (4): 375–8. PMID 10207887. S2CID 12362848. doi:10.1038/7933.
- ^ Jacobsen MT, Fairhead M, Fogelstrand P, Howarth M. Amine Landscaping to Maximize Protein-Dye Fluorescence and Ultrastable Protein-Ligand Interaction. Cell Chem Biol. August 2017, 24 (8): 1040–1047. PMC 5563079
 . PMID 28757182. doi:10.1016/j.chembiol.2017.06.015
. PMID 28757182. doi:10.1016/j.chembiol.2017.06.015  .
.
- ^ Voss EW J, Workman CJ, Mummert ME. Detection of protease activity using a fluorescence-enhancement globular substrate. BioTechniques. February 1996, 20 (2): 286–291. PMID 8825159. doi:10.2144/96202rr06. 温哥华格式错误 (帮助)
- ^ Moens P. Fluorescence Resonance Energy Transfer spectroscopy. [July 14, 2012]. (原始内容存档于2016-07-26).
- ^ 国际纯化学和应用化学联合会,化学术语概略,第二版。(金皮书)(1997)。在线校正版: (2006–) "Dexter excitation transfer (electron exchange excitation transfer)"。doi:10.1351/goldbook.D01654
- ^ Dexter Energy Transfer. chemwiki.ucdavis.edu. 2 October 2013 [8 July 2014]. (原始内容存档于2014-07-14).
- ^ 国际纯化学和应用化学联合会,化学术语概略,第二版。(金皮书)(1997)。在线校正版: (2006–) "Dexter excitation transfer (electron exchange excitation transfer)"。doi:10.1351/goldbook.D01654
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.




![{\displaystyle k_{ET}\varpropto P^{2}J\mathrm {exp} \left[{\frac {-2r}{L))\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/aea19637dfff289c79d07f915f5e55d34223ff2f)
