碳金属化反应
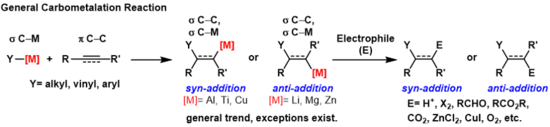
碳金属化反应(英语:carbometalation)是任何过程中发生以下事件的反应:一个碳-金属键与一个碳-碳π键反应,并产生一个新的碳-碳σ键与一个碳-金属σ键[1]。 生成的碳金属键可以进行进一步的碳金属化反应(低聚或聚合,请参见齐格勒-纳塔催化剂聚合),也可以使其与各种亲电体试剂反应,包括卤化试剂、羰基化合物、氧和无机盐,从而生成不同的有机金属试剂。碳金属化反应可以在炔烃和烯烃上被进行,以分别形成具有高几何纯度或对映选择性的产物。
碳铝化
[编辑]
碳铝化反应最通常被二氯二茂锆(或相关催化剂)催化。某些碳铝化是被用二氯二茂钛复合物进行的[1] 。该反应有时称为烯烃的Zr催化不对称碳铝化(ZACA)或炔烃的Zr催化甲基铝化(ZMA)[2] 。
用于该转化的最常见的三烷基铝试剂是三甲基铝,三乙基铝,有时还有三异丁基铝。当使用具有β-氢化物的三烷基铝试剂时,消除反应和氢铝反应成为竞争过程。
碳锂化
[编辑]
碳锂化是在碳-碳pi键上添加有机锂试剂。用于该转化的有机锂试剂可以是商用的(例如正丁基锂),也可以通过去质子化或卤化锂交换生成[3][4]。碳锂化的分子间的和分子内的例子都存在,可被用于合成以产生复杂性。
碳镁化和碳锌化
[编辑]由于格氏试剂(有机镁试剂)和有机锌试剂的降低的亲核性,因此通常仅在活化或应变的烯烃和炔烃上观察到非催化的碳镁化和碳锌化反应[5]。
碳钯化
[编辑]碳钯化可以是一个描述由钯催化剂催化的反应的基本步骤(沟吕木-赫克反应)[6],并且也可以指与钯催化剂的碳金属化反应(烯烃双官能化[7] ,加氢官能化[8][9]) 或还原性[10]))
参考文献
[编辑]- ^ 1.0 1.1 Negishi, Ei-ichi; Tan, Ze, Diastereoselective, Enantioselective, and Regioselective Carboalumination Reactions Catalyzed by Zirconocene Derivatives, Metallocenes in Regio- and Stereoselective Synthesis: -/-, Topics in Organometallic Chemistry (Springer Berlin Heidelberg), 2005: 139–176, ISBN 9783540314523, doi:10.1007/b96003
- ^ Xu, Shiqing; Negishi, Ei-ichi. Zirconium-Catalyzed Asymmetric Carboalumination of Unactivated Terminal Alkenes. Accounts of Chemical Research. 2016-10-18, 49 (10): 2158–2168. ISSN 0001-4842. PMID 27685327. doi:10.1021/acs.accounts.6b00338.
- ^ O’Shea, Donal F.; Hogan, Anne-Marie L. Synthetic applications of carbolithiation transformations. Chemical Communications. 2008-08-18, (33): 3839–3851 [2020-06-18]. ISSN 1364-548X. PMID 18726011. doi:10.1039/B805595E. (原始内容存档于2020-07-27).
- ^ García, Graciela V.; Nudelman, Norma Sbarbati. Tandem Reactions Involving Organolithium Reagents. A Review. Organic Preparations and Procedures International. 2009-02-11, 35 (5): 445–500. doi:10.1080/00304940309355860.
- ^ Yorimitsu, Hideki; Murakami, Kei. Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation. Beilstein Journal of Organic Chemistry. 2013-02-11, 9 (1): 278–302. ISSN 1860-5397. PMC 3596116
 . PMID 23503106. doi:10.3762/bjoc.9.34.
. PMID 23503106. doi:10.3762/bjoc.9.34.
- ^ Negishi, Ei-ichi; Copéret, Christophe; Ma, Shengming; Liou, Show-Yee; Liu, Fang. Cyclic Carbopalladation. A Versatile Synthetic Methodology for the Construction of Cyclic Organic Compounds. Chemical Reviews. January 1996, 96 (1): 365–394. ISSN 0009-2665. PMID 11848757. doi:10.1021/cr950020x.
- ^ Sigman, Matthew S.; Jensen, Katrina H. Mechanistic approaches to palladium-catalyzed alkene difunctionalization reactions. Organic & Biomolecular Chemistry. 2008-10-30, 6 (22): 4083–4088. ISSN 1477-0539. PMC 2656348
 . PMID 18972034. doi:10.1039/B813246A.
. PMID 18972034. doi:10.1039/B813246A.
- ^ Engle, Keary M.; McAlpine, Indrawan; Marsters, Rohan P.; Wang, Fen; He, Mingying; Yang, Shouliang; Gallego, Gary M.; Yang, Kin S.; Hill, David E. Palladium(II)-catalyzed γ-selective hydroarylation of alkenyl carbonyl compounds with arylboronic acids. Chemical Science. 2018-11-14, 9 (44): 8363–8368. ISSN 2041-6539. PMC 6247822
 . PMID 30542583. doi:10.1039/C8SC03081B.
. PMID 30542583. doi:10.1039/C8SC03081B.
- ^ O’Duill, Miriam L.; Matsuura, Rei; Wang, Yanyan; Turnbull, Joshua L.; Gurak, John A.; Gao, De-Wei; Lu, Gang; Liu, Peng; Engle, Keary M. Tridentate Directing Groups Stabilize 6-Membered Palladacycles in Catalytic Alkene Hydrofunctionalization. Journal of the American Chemical Society. 2017-11-08, 139 (44): 15576–15579. ISSN 0002-7863. PMC 6002750
 . PMID 28972751. doi:10.1021/jacs.7b08383.
. PMID 28972751. doi:10.1021/jacs.7b08383.
- ^ Gurak, John A.; Engle, Keary M. Practical Intermolecular Hydroarylation of Diverse Alkenes via Reductive Heck Coupling. ACS Catalysis. 2018-10-05, 8 (10): 8987–8992. PMC 6207086
 . PMID 30393575. doi:10.1021/acscatal.8b02717.
. PMID 30393575. doi:10.1021/acscatal.8b02717.
| |||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
