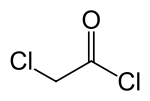
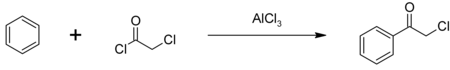氯乙酰氯
| 氯乙酰氯 | |
|---|---|
| |

| |
| IUPAC名 Chloroacetyl chloride | |
| 英文名 | Chloroacetyl chloride |
| 识别 | |
| CAS号 | 79-04-9 |
| PubChem | 6577 |
| ChemSpider | 13856283 |
| SMILES |
|
| InChI |
|
| InChIKey | VGCXGMAHQTYDJK-UHFFFAOYAB |
| EINECS | 201-171-6 |
| KEGG | C14859 |
| 性质 | |
| 化学式 | C2H2Cl2O |
| 摩尔质量 | 112.94 g·mol−1 |
| 外观 | 无色至黄色液体 |
| 密度 | 1.42 g/mL |
| 熔点 | -22 °C(251 K) |
| 沸点 | 106 °C(379 K) |
| 溶解性(水) | 反应 |
| 蒸气压 | 19 mmHg (20°C)[1] |
| 危险性 | |
| 欧盟分类 | |
| 闪点 | 不可燃 |
| PEL | 无[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
氯乙酰氯是氯乙酸的酰氯衍生物,化学式为CH2ClCOCl。它是一种双官能团化合物,作为合成砌块(building block)使用。
制备
工业上,氯乙酰氯可以通过二氯甲烷的羰基化反应制备,或者是1,1-二氯乙烯的氧化反应,也可以利用烯酮的氯化反应得到。[2]其它方法还有氯乙酸和氯化亚砜、五氯化磷或光气的反应。
化学性质
氯乙酰氯作为一种双官能团化合物,其酰氯端可以很容易地形成酯[3]或酰胺,另一端可以形成胺等官能团。例如氯乙酰氯在利多卡因的合成中的使用:[4]
应用
氯乙酰氯的主要用途是作为甲草胺和丁草胺生产过程中的中间体,每年估计使用1亿磅。还有一部分氯乙酰氯用于生产2-氯苯乙酮(苯乙酰氯),另一种可用于生产催泪瓦斯的中间体。[2]以氯化铝为催化剂,苯的傅-克酰基化反应可以制备2-氯苯乙酮:[5]
安全
和其它酰氯一样,氯乙酰氯可以和其他质子化合物如胺、醇或水反应,生成刺激性的氯化氢气体。
美国职业安全与健康管理局没有设定规定的允许暴露的限值,但美国国家职业安全卫生研究所设立的标准是每天工作8小时,建议曝露限值不超过0.05 ppm。[6]
参考文献
- ^ 1.0 1.1 NIOSH Pocket Guide to Chemical Hazards. #0120. NIOSH.
- ^ 2.0 2.1 Paul R. Worsham. 15. Halogenated Derivatives. Zoeller, Joseph R.; Agreda, V. H. (编). Acetic acid and its derivatives (Google Books excerpt). New York: M. Dekker. 1993: 288–298. ISBN 0-8247-8792-7.
- ^ Robert H. Baker and Frederick G. Bordwell (1955). "tert-Butyl acetate". Org. Synth.; Coll. Vol. 3.
- ^ T. J. Reilly. The Preparation of Lidocaine. J. Chem. Ed. 1999, 76 (11): 1557 [2017-08-08]. doi:10.1021/ed076p1557. (原始内容存档于2009-08-07).
- ^ Nathan Levin and Walter H. Hartung (1955). "ω-Chloroisonitrosoacetophenone". Org. Synth.; Coll. Vol. 3: 191.
- ^ NIOSH Pocket Guide to Chemical Hazards. Centers for Disease Control and Prevention. 2011 [2017-08-08]. (原始内容存档于2017-08-09).
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.