氟硅酸镁
| 氟硅酸镁 | ||
|---|---|---|
|
| ||
| IUPAC名 magnesium hexafluorosilicon(2-) | ||
| 英文名 | Magnesium fluorosilicate | |
| 别名 | 硅氟化镁 | |
| 识别 | ||
| CAS号 | 12449-55-7 16949-65-8 18972-56-0(六水) | |
| PubChem | 61861 | |
| SMILES |
| |
| 性质 | ||
| 化学式 | F6MgSi | |
| 摩尔质量 | 166.380919 g·mol⁻¹ | |
| 外观 | 白色无臭固体(六水)[1] | |
| 密度 | 1.79g/cm3[1] | |
| 熔点 | 120℃ (分解)[2] | |
| 溶解性(水) | 六水合物可溶[1] | |
| 溶解性 | 可溶于稀酸[2] 不溶于酒精[2] | |
| 危险性 | ||
| 主要危害 | 有毒 | |
| 致死量或浓度: | ||
LD50(中位剂量)
|
200 mg/kg(豚鼠,口服)[3] | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
氟硅酸镁(Magnesium fluorosilicate),别名硅氟化镁,化学式MgSiF6。一般为六水合物(MgSiF6·6H2O)形式,相对分子质量274.48。
性质
[编辑]六水合物为白色无气味结晶。[1]该结晶难潮解而可风化,风化过程会失去结晶水。相对密度1.788,熔点120°C(分解)。在80°C以上时会脱水分解,同时释放气体四氟化硅。该结晶在水中溶解度高,溶解后可得酸性溶液。此外还可在稀酸中溶解,在氢氟酸中难溶解,不溶于醇。与碱发生化学反应时,其反应物产物包括二氧化硅及相应的氟化物。该物质具有毒性。
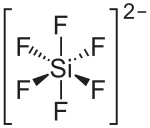
氟硅酸镁是单斜晶系的,[2]不过其它结构也是已知的。[4][5][6]
用途
[编辑]在制作混凝土时,可以用于改善其强度与硬度的硬化剂,同时也是一种防水剂。[7]此外在硅石表面处理中,可用作氟风化处理。由于毒性可以杀虫,亦可用作织物防虫,或者作为杀虫剂的活性成分之一。[8]此外还可以在陶瓷制作中使用。
制备
[编辑]净化后的氟硅酸溶液在反应器中用菱苦土粉悬浮液中和至pH=3~4时,即得氟硅酸镁溶液。再经过滤、浓缩结晶、离心分离、干燥,得到氟硅酸镁成品。
氟硅酸镁也可以由镁化合物和氢氟酸在二氧化硅下反应而成。[9]
参考文献
[编辑]- ^ 1.0 1.1 1.2 1.3 Datenblatt Magnesium hexafluorosilicate hexahydrate, 98% bei AlfaAesar, abgerufen am 2016-11-23.
- ^ 2.0 2.1 2.2 2.3 R. Blachnik, [《氟硅酸镁》在Google Books的内容。 Taschenbuch für Chemiker und Physiker Band 3: Elemente, anorganische Verbindungen und Materialien, Minerale], Springer-Verlag. 2013: pp. 562, (德文)
- ^ Michael Ash, Irene Ash, [《氟硅酸镁》在Google Books的内容。 Handbook of Preservatives], Synapse Info Resources. 2004: pp. 843, (德文)
- ^ Peter G. Skrylnik, Albert M. Ziatdinov: Incommensurate Phases of the MgSiF6-6H2O Crystals: EPR and Group-Theoretical Studies. In: Applied Magnetic Resonance. 45, 2014, S. 623, doi:10.1007/s00723-014-0542-6.
- ^ R. Hrabański, V. Kapustianik, V. Kardash, S. Sveleba: Birefringent and piezooptic properties of (MgSiF6) - 6H2O crystals. In: Physica Status Solidi. 142, 1994, S. 509, doi:10.1002/pssa.2211420225.
- ^ S. Syoyama, K. Osaki: An X-ray study of the low-temperature form of MgSiF6.6H2O and the relation between the crystal lattices of low- and high-temperature forms. In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 28, S. 2626, doi:10.1107/S0567740872006624.
- ^ Richard P. Pohanish, [《氟硅酸镁》在Google Books的内容。 Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens], William Andrew. 2011: pp. 3039, (德文)
- ^ Ralf Steudel, [《氟硅酸镁》在Google Books的内容。 Chemie der Nichtmetalle Von Struktur und Bindung zur Anwendung], Walter de Gruyter. 2008: pp. 282, (德文)
- ^ Umweltbundesamt: Merkblatt über die besten verfügbaren Techniken für die Herstellung Anorganischer Grundchemikalien: Ammoniak, Säuren und Düngemittel, August 2007 (页面存档备份,存于互联网档案馆)
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

![{\displaystyle \mathrm {MgO+SiO_{2}+6\ HF\longrightarrow Mg[SiF_{6}]+3\ H_{2}O} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/897f0e068141bab15026c5edc572e5eb46a0d0f5)
