五氟碲酸
| 五氟碲酸 | |||
|---|---|---|---|
| |||
| IUPAC名 Pentafluoroorthotelluric acid | |||
| 英文名 | Teflic acid | ||
| 识别 | |||
| CAS号 | 57458-27-2 | ||
| PubChem | 15243876 | ||
| ChemSpider | 10331773 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | OAOSLENTGBMCNC-UHFFFAOYAO | ||
| 性质 | |||
| 化学式 | HF5OTe | ||
| 摩尔质量 | 239.6 g·mol⁻¹ | ||
| 外观 | 无色固体 | ||
| 熔点 | 39.1 °C(312 K) | ||
| 沸点 | 59.7 °C(333 K) | ||
| 危险性 | |||
GHS危险性符号
| |||
| GHS提示词 | 危险 | ||
| H-术语 | H314, H318 | ||
| P-术语 | P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | ||
| 主要危害 | 腐蚀性,有毒 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
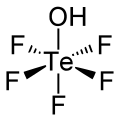
五氟碲酸是一种无机化合物,化学式为HOTeF5。它是原碲酸(Te(OH)6)的五个羟基被氟取代的产物,具有强酸性。它有稍微扭曲的八面体几何构型。
制备
[编辑]五氟碲酸最初于1964年由恩格尔布雷希特和斯拉德基偶然发现,他们试图通过碲酸钡和氟磺酸的反应制备二氟二氧化碲(TeO2F2),但未得到期望的产物,得到了一些挥发性的碲化合物,其中包含五氟碲酸(HOTeF5)。[1]
后来,霍里曼等人通过氟磺酸和碱式碲酸钡的反应来制备五氟碲酸:[2]
- 5HOSO2F + BaO2Te(OH)4 → HOTeF5 + 4 H2SO4 + BaSO4
- TeF6 + H2O → HOTeF5 + HF
性质
[编辑]五氟碲酸和原碲酸混合加热,可以得到顺式及反式的(HO)2TeO4和(HO)xTeF6−x。[4]它和NF4HF2在低温反应,可以得到FOTeF5:[5]
- HOTeF5 + NF4HF2 → FOTeF5 + NF3 + 2 HF
- HOTeF5 + ClX → ClOTeF5 + HX (X=F或OSO2F)
它的无机或有机盐是已知的[7][8],它也可以形成酸式盐,其中含有[H(OTeF5)2]−。[9]
它和三乙基铝、氯甲烷反应,可以得到[(CH3)2Cl][Al(OTeF5)4],它是一种很强的甲基化试剂。[10]
- 8 HOTeF5 + Al2(C2H5)6 + 2 CH3Cl → 2 [(CH3)2Cl][Al(OTeF5)4] + 2 HCl + 6 C2H6
参考文献
[编辑]- ^ Engelbrecht, A.; Sladky, F. Pentafluoro-orthotellursäure, HOTeF5. Angewandte Chemie (Wiley). 1964-05-07, 76 (9): 379–380. ISSN 0044-8249. doi:10.1002/ange.19640760912 (德语).
- ^ Wiberg, Egon. Inorganic chemistry. San Diego Berlin New York: Academic Press De Gruyter. 2001. ISBN 0-12-352651-5. OCLC 48056955 (英语).
- ^ Ushakov, O. S.; Kalashnikov, A. L.; Matyukha, V. A.; Smagin, A. A.; Ushakova, T. V.; Kozlova, R. D. Study of products of hydrolysis of tellurium hexafluoride. Izvestiya Vysshikh Uchebnykh Zavedenii, Tsvetnaya Metallurgiya. 2003, (6): 25–28. ISSN 0021-3438 (俄语).
- ^ Pötter, Brigitte; Lentz, Dieter; Pritzkow, Hans; Seppelt, Konrad. gem-Bis(halogenoxy)-Verbindungen auscis- undtrans- Tetrafluorodioxotellur(VI)säure, (HO)2TeF4. Angewandte Chemie (Wiley). 1981, 93 (12): 1095–1096. ISSN 0044-8249. doi:10.1002/ange.19810931227 (德语).
- ^ Schack, Carl J.; Wilson, William W.; Christe, Karl O. Synthesis and characterization of tellurium fluoride hypofluorite (TeF5OF). Inorganic Chemistry (American Chemical Society (ACS)). 1983, 22 (1): 18–21. ISSN 0020-1669. doi:10.1021/ic00143a005 (英语).
- ^ Schack, Carl J.; Christe, Karl O. New syntheses of pentafluorotellurium hypochlorite. Journal of Fluorine Chemistry (Elsevier BV). 1982, 21 (3): 393–396. ISSN 0022-1139. doi:10.1016/s0022-1139(00)81525-0 (英语).
- ^ Miller, P. K.; Abney, K. D.; Rappe, A. K.; Anderson, O. P.; Strauss, S. H. Electronic and molecular structure of pentafluorooxotellurate(1-). Inorganic Chemistry (American Chemical Society (ACS)). 1988, 27 (13): 2255–2261. ISSN 0020-1669. doi:10.1021/ic00286a010 (英语).
- ^ Crossman, Martin C.; Hope, Eric G.; Saunders, Graham C. Cyclopentadienyl metal teflate (OTeF5) complexes. Journal of the Chemical Society, Dalton Transactions (Royal Society of Chemistry (RSC)). 1996, (4): 509. ISSN 0300-9246. doi:10.1039/dt9960000509 (英语).
- ^ Strauss, Steven H.; Abney, Kent D.; Anderson, Oren P. An unusual hydrogen bond between oxygen atoms: preparation and characterization of tetrabutylammonium hydrogen bis(pentafluorooxotellurate). Inorganic Chemistry (American Chemical Society (ACS)). 1986, 25 (16): 2806–2812. ISSN 0020-1669. doi:10.1021/ic00236a030 (英语).
- ^ Hämmerling, Sebastian; Thiele, Günther; Steinhauer, Simon; Beckers, Helmut; Müller, Carsten; Riedel, Sebastian. A Very Strong Methylation Agent: [Me2Cl][Al(OTeF5)4]. Angewandte Chemie International Edition (Wiley). 2019-07-15, 58 (29): 9807–9810. ISSN 1433-7851. doi:10.1002/anie.201904007 (英语).
拓展阅读
[编辑]
- Herbers, S.; Obenchain, D. A.; Kraus, P.; Wachsmuth, D.; Grabow, J.-U. Blurring out hydrogen: The dynamical structure of teflic acid. The Journal of Chemical Physics (AIP Publishing). 2018-05-21, 148 (19): 194307. ISSN 0021-9606. doi:10.1063/1.5027487 (英语).
- The Chemistry of Highly Electronegative OTeF5 Group (PDF). Jayantha Amarasekera. 1986-05-06 [2021-08-14]. (原始内容 (PDF)存档于2022-02-10).
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.