氧化亚铁
| 氧化亚铁 | |
|---|---|

| |

| |
| IUPAC名 Iron(II) oxide 氧化铁(II) | |
| 别名 | 氧化亚铁 (ferrous oxide) 一氧化铁 (iron monoxide) |
| 识别 | |
| CAS号 | 1345-25-1 |
| ChemSpider | 14237 |
| InChIKey | UQSXHKLRYXJYBZ-WPTVXXAFAB |
| Gmelin | 13590 |
| 性质 | |
| 化学式 | FeO |
| 摩尔质量 | 71.85 g·mol⁻¹ |
| 密度 | 5.7 g/cm³ |
| 熔点 | 1370 °C (1643.15 K) |
| 沸点 | 3414 °C (3687.15 K) |
| 溶解性(水) | 不溶 |
| 危险性 | |
| 主要危害 | 引火 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
氧化亚铁,是铁的氧化物之一。其外观呈蓝灰色粉末,化学式为FeO,由氧化态为II价的铁与氧共价结合。它的矿物形式为方铁矿。氧化亚铁经常容易与铁锈混淆,但铁锈的主要成分为水合氧化铁。氧化亚铁属于非整比化合物,其中铁和氧元素的比例会发生变化,范围从Fe0.84O到Fe0.95O。[1]
制备
FeO可以在隔绝空气条件下加热草酸亚铁制得。
整比的FeO可以通过加热Fe0.95O与金属铁,在770℃和36kbar压力条件下生成。[3]
FeO也可以在900℃条件下通过氧化铁与一氧化碳反应(在还原焰中加热氧化铁)得到。
相关反应
FeO在低于575℃的条件下具有热不稳定性,可以歧化生成金属铁和Fe3O4[1]:
FeO可与一些元素单质反应,而被还原为铁单质:
结构
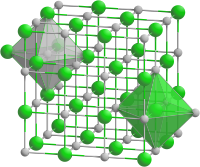
__ Fe2+ __ O2−
氧化亚铁属于立方晶系(类似于氯化钠晶体结构),每个铁原子周围连接着6个氧原子形成八面体型配位,每个氧原子周围也以同样的情况连接着6个铁原子。出现非整比化合物的原因在于,由于二价铁很容易被氧化为三价铁,导致FeO中少量二价铁被替换为三价铁,占据晶格中的四面体空隙。[3]
用途
参考资料
- ^ 1.0 1.1 Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements 2nd. Oxford:Butterworth-Heinemann. 1997. ISBN 0-7506-3365-4.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999). Advanced Inorganic Chemistry (6th Edn.) New York:Wiley-Interscience. ISBN 0-471-19957-5
- ^ 3.0 3.1 3.2 Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford University Press ISBN 0-19-855370-6
参见
外部链接
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.








