Sukcinska kiselina
Izvor: Wikipedija
| Sukcinska kiselina | |||
|---|---|---|---|
| |||

| |||

| |||
| IUPAC ime |
| ||
| Drugi nazivi | etan-1,2-dikarboksilna kiselina | ||
| Identifikacija | |||
| CAS registarski broj | 110-15-6 | ||
| PubChem[1][2] | 1110 | ||
| ChemSpider[3] | 1078 | ||
| UNII | AB6MNQ6J6L | ||
| DrugBank | DB00139 | ||
| ChEBI | 15741 | ||
| ChEMBL[4] | CHEMBL576 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
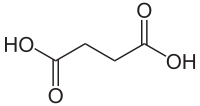
| Molekulska formula | C4H6O4 | ||
| Molarna masa | 118.09 g mol−1 | ||
| Gustina | 1,56 g/cm3[5] | ||
| Tačka topljenja |
184 °C, 457 K, 363 °F ([5]) | ||
| Tačka ključanja |
235 °C, 508 K, 455 °F ([5]) | ||
| Rastvorljivost u vodi | 58 g/L (20 °C)[5] | ||
| pKa | pKa1 = 4.2 pKa2 = 5.6 | ||
| Opasnost | |||
| Tačka paljenja | 206 °C[5] | ||
| Srodna jedinjenja | |||
| Drugi anjoni | natrijum sukcinat | ||
| Srodna karboksilna kiselina | propionska kiselina malonska kiselina buterna kiselina maleinska kiselina tartaratna kiselina fumarna kiselina pentanska kiselina glutarna kiselina | ||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Sukcinska kiselina (butandionska kiselina, ćilibarna kiselina) je dikarboksilna kiselina. Sukcinska kiselina je bela čvrsta materija, bez mirisa. Ona je diprotična kiselina. Sukcinat učestvuje u ciklusu limunske kiseline, procesu kojim se oslobađa energija. Njeno ime je izvedeno iz lat. succinum, sa značanjem ćilibar, iz čega se ova kiselina može dobiti.
Ćilibarna kiselina je originalno dobijena iz ćilibara mlevenjem i destilacijom u pešćanom kupatilu. U prošlosti je uglavnom spoljašnje korištena za tretman reumatičnih bolova, i unutrašnje za lečenje okorelih slučajeva gonoreje.
Sukcinska kiselina se proizvodi na nekoliko načina. Česti industrijski putevi su hidrogenacija maleinske kiseline, oksidacija 1,4-butanediola, i karbonilacija etilen glikola.[6]
Sukcinska kiselina se može konvertovati u fumarnu kiselinu oksidacijom. Dietil estar je supstrat u Stobijevoj kondenzaciji. Dehidratacija sukcinske kiseline proizvodi sukcinski anhidrid.[7]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ 5,0 5,1 5,2 5,3 5,4 Record in the GESTIS Substance Database from the IFA
- ↑ Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. DOI:10.1002/14356007.a08_523
- ↑ Louis F. Fieser, E. L. Martin, R. L. Shriner, and H. C. Struck (1932), „Succinic Anhydride”, Organic Syntheses 12: 66; Coll. Vol. 2: 560.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
