Nitrogen oksida
Nitrogen oksida boleh merujuk kepada sebatian dedua oksigen dan nitrogen, atau campuran sebatian seperti:
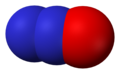
- Nitrik oksida, juga dikenali sebagai nitrogen monoksida, (NO), nitrogen(II) oksida
- Nitrogen dioksida (NO2), nitrogen(IV) oksida
- Nitrus oksida (N2O), nitrogen(−I,III) oksida
- Nitrosil azida (N4O), nitrogen(−I,0,I,II) oksida
- Dinitrogen trioksida (N2O3), nitrogen(II,IV) oksida
- Dinitrogen tetroksida (N2O4), nitrogen(IV) oksida
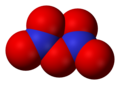
- Dinitrogen pentoksida (N2O5), nitrogen(V) oksida
- Radikal bebas nitrat(NO3•), nitrogen(VI) oksida, ialah sejenis radikal bebas
- Trinitramida (N(NO2)3), nitrogen(0,IV) oksida
Sebatian NO dan NO2 ialah radikal. Tambahan pula, terdapat beberapa anion nitrogen oksida. Yang paling stabil di antaranya ialah anion nitrat:
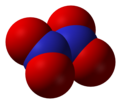
- Nitrat (NO3-), trioxonitrate(V) ion
tetapi nitrogen oxoanion yang lain termasuk nitrit, peroxonitrite, trioxodinitrate (hyponitrate), dan nitroxylate.
Dalam kimia atmosfera, pencemaran udara, dan bidang yang berkaitan, nitrogen oksida merujuk secara khususnya kepada NOx (NO dan NO2).[1][2]
Hanya tiga pertama sebatian ini boleh diasingkan pada suhu bilik. N2O3, N2O4, dan N2O5 semua mengurai dengan cepat pada suhu bilik. NO3, N4O, dan N(NO2)3 sangat reaktif.
N2O stabil dan agak tidak reaktif pada suhu bilik, manakala NO dan NO2 agak reaktif namun agak stabil apabila diasingkan.
-
Nitrik oksida, NO -
Nitrogen dioksida, NO2 -
Nitrus oksida, N2O -
Dinitrogen trioksida, N2O3 -
Dinitrogen tetroksida, N2O4 -
Dinitrogen pentoksida, N2O5 -
Trinitramida, N(NO2)3
Rujukan
[sunting | sunting sumber]- ^ United States Clean Air Act, 42 U.S.C. § 7602
- ^ Seinfeld, John H.; Pandis, Spyros N. (1997), Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, Wiley-Interscience, ISBN 0-471-17816-0
| Tahap pengoksidaan bercampur | |
|---|---|
| Tahap pengoksidaan +1 |
|
| Tahap pengoksidaan +2 |
|
| Tahap pengoksidaan +3 |
|
| Tahap pengoksidaan +4 |
|
| Tahap pengoksidaan +5 |
|
| Tahap pengoksidaan +6 |
|
| Tahap pengoksidaan +7 |
|
| Tahap pengoksidaan +8 |
|
| Oksida berkaitan |
|
Oksida disusun berdasarkan tahap pengoksidaan.
Kategori:Oksida | |
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.