Amonija jons
| Amonija jons | |
|---|---|
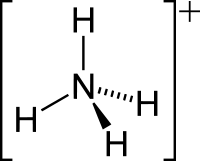 Amonija jona struktūrformula   Amonija jona modeļi | |
| Citi nosaukumi | amonijs |
| CAS numurs | 14798-03-9 |
| Ķīmiskā formula | NH4+ |
| Molmasa | 18,04 g/mol |
Amonija jons jeb vienkārši amonijs ir pozitīvi lādēts vairākatomu jons ar formulu NH4+. Ja amonija joniem pievienojas anjoni (negatīvi lādēti joni), veidojas amonija sāļi, kas pieder pie tā dēvētajiem "onija" sāļiem.
Amonija jonam ir tetraedriska forma, kur centrā ir slāpekļa atoms, kam apkārt izvietojušies ūdeņraža atomi. Jona izmērs ir 1,43 Å.[1]

Amonija joni viegli veidojas, amonjakam reaģējot ar skābēm:
- NH3 + HCl → NH4Cl
Ūdens šķīdumos amonija sāļi disociē:
- NH4Cl ⇄ NH4+ + Cl−
Ūdeņraža atomi amonija jonā var tikt aizvietoti ar citiem atomiem, piemēram, fluora atomiem (tetrafluoramonija katjons [NF4]+). Tie var aizvietoties arī ar organiskām grupām. Ja visi ūdeņraža atomi amonija jonā tiek aizvietoti ar metilgrupām, rodas tetrametilamonija jons. Atšķirībā no amonija hidroksīda, kas pastāv (nosacīti) tikai ūdens šķīdumos, tetrametilamonija hidroksīds ir samērā stabila viela.
Atsauces
[labot šo sadaļu | labot pirmkodu]| Vikikrātuvē par šo tēmu ir pieejami multivides faili. Skatīt: amonija jons |
- ↑ Nails Ahmetovs. Neorganiskā ķīmija. Rīga : Zvaigzne, 1978, 371. lpp.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
