Zaatwater
Dit artikel is gesjreve (of begós) in 't Mofers. Laes hie wie v'r mit de versjillende saorte Limburgs ómgaon.
Zaatwater (mitte klemtoean op zaat-) is water mit 'n hoger saliniteit (zaatgehaldje) es brakwater, in feite betruf 't hie 'n oplossing van zaten in 't water. Me stèlj hiebie inne regel 'n zaatoplossing van minstes 1% (massa-aandeil). Water mit 'n klènder gehaldje neump me brakwater, en water mit minder es 0,1% zaat neump me zeutwater. 't Meiste zaatwater oppe aerd vindj me trögk in 't zieëwater vanne oceane; dit vörmp drek ouch de grótste watermassa oppe plenieët. 't Gemiddeldj zaatgehaldje vanne zieëje ligk róndje 3,5%. 't Hoogste zaatgehaldje (44,2%) vindj me in 't hypersalien water van 't Don Juanmaer op Antarctica; dit maer bevatj aevel veural kalkchloried inplaats natriumchloried.
't Zaatgehaldje van water kan me maete mit 'ne salinomaeter.
Eigesjappe
[bewirk | brón bewèrke]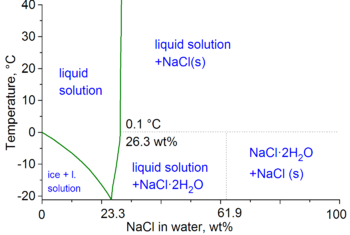

Zaatwater haet anger eigesjappen es zeutwater. De voesregel hiebie is det wie zater 't water is, wie hoger 't driefvermeugen is en wie minder snel 't bevruus.
| NaCl (gewichspercent) | Vreerpuntj (°C) | Dichheid (g/cm3) | Braekingsindex nie 589 nm | Viscositeit (cP) |
|---|---|---|---|---|
| 0 | 0 | 0.99984 | 1.3330 | 1.002 |
| 0.5 | −0.3 | 1.0018 | 1.3339 | 1.011 |
| 1 | −0.59 | 1.0053 | 1.3347 | 1.02 |
| 2 | −1.19 | 1.0125 | 1.3365 | 1.036 |
| 3 | −1.79 | 1.0196 | 1.3383 | 1.052 |
| 4 | −2.41 | 1.0268 | 1.3400 | 1.068 |
| 5 | −3.05 | 1.0340 | 1.3418 | 1.085 |
| 6 | −3.7 | 1.0413 | 1.3435 | 1.104 |
| 7 | −4.38 | 1.0486 | 1.3453 | 1.124 |
| 8 | −5.08 | 1.0559 | 1.3470 | 1.145 |
| 9 | −5.81 | 1.0633 | 1.3488 | 1.168 |
| 10 | −6.56 | 1.0707 | 1.3505 | 1.193 |
| 12 | −8.18 | 1.0857 | 1.3541 | 1.25 |
| 14 | −9.94 | 1.1008 | 1.3576 | 1.317 |
| 16 | −11.89 | 1.1162 | 1.3612 | 1.388 |
| 18 | −14.04 | 1.1319 | 1.3648 | 1.463 |
| 20 | −16.46 | 1.1478 | 1.3684 | 1.557 |
| 26 | −19.18 | 1.193 | 1.3721 | 1.676 |
Bie 100 °C kan verzadig pekel van natriumchloried zoeaget 28% zaat bevatte (gewichspercentaasj); bie 0°C is det zoea 26%.
De thermische geleibaarheid van zieëwater (3,5% opgelos zaat) 0.6 W/mK bie 25 °C. De thermische geleibaarheid nump aaf es 't zaatgehaldje toenump en nump juus toe bie toenummendje temperatuur.
Indeiling en toepassinge
[bewirk | brón bewèrke]Zaatwater wuuert döks in drie categoriejen ingedeildj:
- Lich zaatwater mit e zaatgehaldje van 1000 toet 3000 deiler de miljoen (ppm) of 1 toet 3 deiler d'n doezjend.
- Maotig zaatwater mit e zaatgehaldje van 3000 toet 10 000 deiler de miljoen of 3 toet 10 deiler d'n doezjend.
- Zwaor zaatwater mit e zaatgehaldje van 10 000 toet 35 000 deiler de miljoen of 10 toet 35 deiler d'n doezjend.
Ter vergelieking: 't zieëwater haet mit en nao 35.000 deiler de miljoen (25 g/l). Zaatwater mit e zaatgehaldje toet 2500 deiler de miljoen wuuert in sómmige gebieden oppe werreld nag gebroek veur 't bespreuje van gewasje, door e tekort aan zat zeutwater. Dit kan op langen termien väöl sjaaj toebringen ane baom enne landjboew.
Inne steriele vorm van 'n zaatwateroplossing wuuert 't ouch väöl toegepas inne genaeskónde.
Zaatwater wuuert ouch gebroek inne industrie, wie smultjzaat en es keulmiddel in wurmdjekrafcentrale.
Óngevieër 4% van 't waterstófgaas det me weltwied perduceerdj wuuert gemaak door gebroek te make van elektrolyse. De mieëderheid van deze waterstóf waat zoea wuuert gemaak is e bieperduk vanne perduksje van chlorien:
- 2 NaCl(aq) + 2 H2O(l) → 2 NaOH(aq) + H2(g) + Cl2(g)
Euverzich
[bewirk | brón bewèrke]| Zeut | Brak | Lich zaat | Maotig zaat | Zwaor zaat | Hypersalien | Pekel |
|---|---|---|---|---|---|---|
| < 0,5 | 0,5-1 | 1-3 | 3-10 | 10-30 | 30-50 | > 50 |
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
