Talium(I) fluorida
| |
| Nama | |
|---|---|
| Nama IUPAC (preferensi)
Talium(I) fluorida | |
| Nama lain
Talium monofluorida
Talo fluorida | |
| Penanda | |
Model 3D (JSmol)
|
|
| 3DMet | (({3DMet))} |
| ChemSpider | |
| Nomor EC | |
PubChem CID
|
|
| Nomor RTECS | (({value))} |
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Sifat | |
| TlF | |
| Massa molar | 223,3817 g/mol |
| Penampilan | Kristal putih |
| Densitas | 8,36 g cm−3 |
| Titik lebur | 327 °C (621 °F; 600 K) |
| Titik didih | 655 °C (1.211 °F; 928 K) (terurai) |
| 78,6 g/100 mL (pada suhu 15 °C)[1] | |
| Kelarutan | Sedikit larut dalam etanol |
| −44,4·10−6 cm3/mol | |
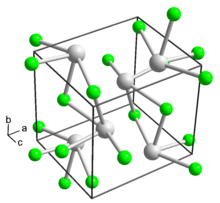
| Struktur | |
| Ortorombik, oP8 | |
| Fmmm, No. 28 | |
| Bahaya[2] | |
| Piktogram GHS |   
|
| Keterangan bahaya GHS | (({value))} |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+310, P304+340, P310, P314, P320, P321, P330, P391, P403+233, P405, P501 | |
| Senyawa terkait | |
Anion lain
|
Talium(I) klorida |
Kation lainnya
|
Galium(III) fluorida Indium(III) fluorida |
Kecuali dinyatakan lain, data di atas berlaku pada suhu dan tekanan standar (25 °C [77 °F], 100 kPa). | |
| Referensi | |
Talium(I) fluorida adalah sebuah senyawa anorganik dengan rumus TlF. Senyawa ini adalah padatan putih yang membentuk kristal ortorombik. Padatan ini sedikit bersifat delikuesen.[1] Ia memiliki struktur kristal natrium klorida (garam batu) yang terdistorsi[3][4] karena adanya pasangan lengai 6s2 pada Tl+.[5]
Garam ini memiliki sifat yang tidak biasa di antara senyawa talium(I) halida karena sangat larut dalam air.[6]
Reaksi
[sunting | sunting sumber]Talium(I) fluorida dapat dibuat melalui reaksi antara talium(I) karbonat dengan asam fluorida.[3]
Referensi
[sunting | sunting sumber]- ^ a b Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, hlm. 407, ISBN 0-8493-8671-3, diakses tanggal 7 Februari 2024
- ^ "399833 Thallium(I) fluoride 99%". Sigma-Aldrich. Diakses tanggal 7 Februari 2024.
- ^ a b Wiberg, Nils; Wiberg, Egon; Holleman, A. F. (2001), Inorganic Chemistry, Academic Press, hlm. 1037, ISBN 0-12-352651-5, diakses tanggal 7 Februari 2024
- ^ Meyer, Gerd; Naumann, Dieter; Wesemann, Lars (2006), Inorganic Chemistry in Focus III, Wiley-VCH, hlm. 21, ISBN 3-527-31510-1, diakses tanggal 7 Februari 2024
- ^ Berastegui, P.; Hull, S. (2000). "The Crystal Structures of Thallium(I) Fluoride". Journal of Solid State Chemistry. 150 (2): 266. Bibcode:2000JSSCh.150..266B. doi:10.1006/jssc.1999.8587.
- ^ Arora, M. G. (2003), P-block Elements, Anmol Publications, hlm. 35, ISBN 81-7488-563-3, diakses tanggal 7 Februari 2024
| Talium(I) |
| ||
|---|---|---|---|
| Talium(III) | |||
Senyawa fluorin | |
|---|---|
| Senyawa biner |
|
| Lain-lain |
|
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
