Karbeno

Kimika organikoan karbeno karbono dibalente bat duen erradikal organiko oso erreaktiboen familiari esaten zaio Karbenorik arruntena :CH2 da, eta metileno deritzo. Bi lotura-elektroi elkarbanatu gabe dituzte karbenoek[1].
Egitura
[aldatu | aldatu iturburu kodea]Bi karbeno-mota daude:
- Singlete karbenoa
- Triplete karbenoa
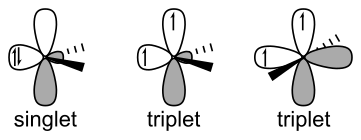
Singlete karbenoak sp2 hibridazioa du elkarbanatu gabeko bi elektroiak p orbital berean parekaturik kokatzen direlako eta laua da. Triplete karbenoa lineala da eta sp hibridazioa du, bi elektroiak parekatu barik daudelarik[2].
Erreaktibitatea
[aldatu | aldatu iturburu kodea]Karbenoen erreaktibitatea motaren araberakoa da, hots, singlete ala triplete den.
Singlete karbenoa bada eta alkenoekiko adizio-erreazioa estereoespezifikoa da. Alegia, cis alkenoak singlete karbenoarekin erreakzionatzen badu, cis-ziklopropanoa lortzen da. Bestalde, trans alkenoa bada, trans-ziklopropanoa lortuko da.
Alabaina, triplete karbenoaren kasuan erreakzioa ez da estereoespezifikoa eta isomeroen nahastea lortzen da[3].

Erabilera
[aldatu | aldatu iturburu kodea]Industrian karbenoen erabilera handiena tetrafluoroetilenoaren produkzioan gertatzen da, hain zuzen ere tefloiaren monomeroa sintetizatzeko. Tetrafluoroetilenoa difluorokarbenoa bitartekoa dela sintetizatzen da.
- CHClF2 → :CF2 + HCl
- 2 :CF2 → F2C=CF2
Erreferentziak
[aldatu | aldatu iturburu kodea]- ↑ Karbeno. «ZT Hiztegi Berria» zthiztegia.elhuyar.eus (Noiz kontsultatua: 2021-05-01).
- ↑ (Ingelesez) Skell, Philip S. & Woodworth, Robert C.. (1956; 78(17);). Structure of Carbene CH2. J. Am. Chem. Soc, 4496-4497 or..
- ↑ (Ingelesez) Morrison, R.T. & Boyd, R.N.. (1992). Organic Chemistry. Prentice Hall, 473-478 or. ISBN 978-0136436690..
Kanpo estekak
[aldatu | aldatu iturburu kodea]Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
