Sulfenyl chloride
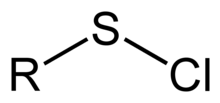
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity R−S−Cl, where R is alkyl[1] or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+. They are used in the formation of RS−N and RS−O bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.
Typically, sulfenyl halides are stabilized by electronegative substituents. This trend is illustrated by the stability of CCl3SCl obtained by chlorination of carbon disulfide.
Preparation

Sulfenyl chlorides are typically prepared by chlorination of disulfides:[2][3] This reaction is sometimes called the Zincke disulfide reaction, in recognition of Theodor Zincke.[4][5]
Some thioethers (R−S−R’) with electron-withdrawing substituents undergo chlorinolysis of a C−S bond to afford the sulfenyl chloride.[6][7]
In a variation on the Reed reaction, sulfur dichloride displaces hydrogen under UV light.[8]
Reactions
Perchloromethyl mercaptan (CCl3SCl) reacts with N−H bonds in the presence of base to give the sulfenamides:
This method is used in the production of the fungicides Captan and Folpet.
Sulfenyl chlorides add across alkenes, for example ethylene:[9]
They undergo chlorination to the trichlorides:[3]
Sulfenyl chlorides react with water and alcohols to give sulfenyl esters (R−S−O−R′):[10]
Route to sulfinyl halides
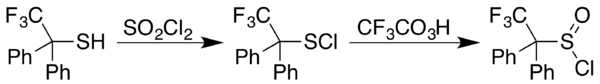
Sulfenyl chlorides can be converted to sulfinyl chlorides (RS(O)Cl). In one approach, the sulfinyl chloride is generated in two steps starting with reaction of a thiol (−SH) with sulfuryl chloride (SO2Cl2). In some cases the sulfenyl chloride results instead, as happens with 2,2,2-trifluoro-1,1-diphenylethanethiol. A trifluoroperacetic acid oxidation then provides a general approach to formation of sulfinyl chlorides from sulfenyl chlorides:[11]
Related compounds
Sulfenyl fluorides and bromides are also known.[12] Simple sulfenyl iodides are unknown because they are unstable with respect to the disulfide and iodine:
Sulfenyl iodides can be isolated as stable compounds if they bear alkyl steric protecting groups as part of a cavity-shaped framework, illustrating the technique of kinetic stabilization of a reactive functionality, as in the case of sulfenic acids.[13]
A related class of compounds are the alkylsulfur trichlorides, as exemplified by methylsulfur trichloride, CH3SCl3.[14]
The corresponding selenenyl halides, R−SeCl, are more commonly encountered in the laboratory. Sulfenyl chlorides are used in the production of agents used in the vulcanization of rubber.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.



![{\displaystyle {\ce {R-SCl + Cl2 -> [R-SCl2]Cl))}](https://wikimedia.org/api/rest_v1/media/math/render/svg/deaf1569a9b4e7730eaea75337aa67878db5868c)



