Uranyl chloride
| |
| Names | |
|---|---|
| IUPAC name
Dichlorodioxouranium
| |
| Other names
Uranium(VI), dichlorodioxy
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.315 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| UO2Cl2 | |
| Molar mass | 340.90 |
| Melting point | Decomposes |
| Boiling point | Decomposes |
| Solubility in other solvents | 320 @ 18C |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
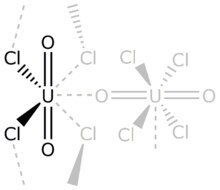
Uranyl chloride refers to inorganic compounds with the formula UO2Cl2(H2O)n where n = 0, 1, or 3. These are yellow-colored salts.
Synthesis and structures
The hydrates are obtained by dissolving uranyl sulfate or uranyl acetate in hydrochloric acid followed by crystallization from concentrated solutions. Depending on the method of drying, one obtains the mono- or the trihydrate. The monohydrate is described as a yellow, sulfur-like powder. It is very hygroscopic.[2] The trihydrate is greenish-yellow. Both hydrates are fluorescent solids that are highly soluble in water.[3]
The anhydrous material can be obtained by the reaction of oxygen with uranium tetrachloride:
- UCl4 + O2 → UO2Cl2 + Cl2
In terms of structures, all three of these compounds feature the uranyl center (trans-UO22+) bound to five additional ligands, which can include (bridging) chloride, water, or another uranyl oxygen.[4][5]
Reactions
The aquo ligands can be replaced by a variety of donors, e.g. THF.[6]
Industrial importance
The company Indian Rare Earths Limited (IREL) has developed a process to extract uranium from the Western and Eastern coastal dune sands of India. After pre-processing with high-intensity magnetic separators and fine grinding, the mineral sands (known as monazite), are digested with caustic soda at about 120 °C (248 °F) and water. The hydroxide concentrate is further digested with concentrated hydrochloric acid to solubilise all hydroxides to form a feed solution composed of chlorides of uranium and other rare earth elements including thorium. The solution is subjected to liquid–liquid extraction with dual solvent systems to produce uranyl chloride and thorium oxalate. The crude uranyl chloride solution is subsequently refined to nuclear grade ammonium diuranate by a purification process involving precipitation and solvent extraction in a nitrate media.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
