Thelephoric acid
| |

| |
| Names | |
|---|---|
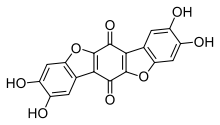
| Preferred IUPAC name
2,3,8,9-Tetrahydroxybenzo[1,2-b:4,5-b′]bis([1]benzofuran)-6,12-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H8O8 | |
| Molar mass | 352.254 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thelephoric acid is a terphenylquinone pigment that is found in several fungi, such as Omphalotus subilludens[1] and Polyozellus multiplex.[2] Thelephoric acid has been shown to inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins (specifically, amyloid precursor protein) in Alzheimer's disease. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects.[3][4] It is derived from atromentin, and its precursor can be from cyclovariegatin. Fragmentation patterns have suggested that polymers of thelephoric acid exists.
References
[edit]- ^ Sullivan G, Garrett RD, Lenehan RF (1971). "Occurrence of atromentin and thelephoric acid in cultures of Clitocybe subilludens". Journal of Pharmaceutical Sciences. 60 (11): 1727–29. doi:10.1002/jps.2600601134. PMID 4332377.
- ^ Ju-Yeon K, Rhee I-K, Lee K-B, Hwang J-S, Yoo I-D, Song K-S (1999). "Thelephoric acid and kynapcin-9 in mushroom Polyozellus multiflex inhibit prolyl endopeptidase in vitro". Journal of Microbiology and Biotechnology. 9 (6): 798–803.
- ^ Hwang JS, Song KS, Kim WG, Lee TH, Koshino H, Yoo ID (1997). "Polyozellin, a new inhibitor of prolyl endopeptidase from Polyozellus multiplex". The Journal of Antibiotics. 50 (9): 773–77. doi:10.7164/antibiotics.50.773. PMID 9360624.
- ^ Nagasawa I, Kaneko A, Suzuki T, Nishio K, Kinoshita K, Shiro M, Koyama K (2014). "Potential anti-angiogenesis effects of p-terphenyl compounds from Polyozellus multiplex". Journal of Natural Products. 77 (4): 963–8. doi:10.1021/np401046z. PMID 24601669.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
