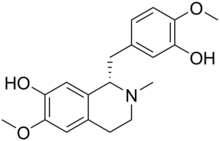
Reticuline
| |
| |
| Names | |
|---|---|
| IUPAC name
3,10-Dimethoxy-8,8a-secoberbine-2,9-diol
| |
| Systematic IUPAC name
(1S)-1-[(3-Hydroxy-4-methoxyphenyl)methyl]-6-methoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.920 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H23NO4 | |
| Molar mass | 329.396 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Reticuline is a chemical compound found in a variety of plants including Lindera aggregata,[1] Annona squamosa,[2] and Ocotea fasciculata (also known as Ocotea duckei).[3]
Reticuline is one of the alkaloids found in opium, and experiments in rodents suggest it possesses potent central nervous system depressing effects.[3] It is the precursor of morphine and many other alkaloids. It is also toxic to dopaminergic neurons causing a form of atypical parkinsonism known as Guadeloupean Parkinsonism.[4]
Metabolism
[edit]3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase uses S-adenosyl methionine and 3'-hydroxy-N-methyl-(S)-coclaurine to produce S-adenosylhomocysteine and (S)-reticuline.
Reticuline oxidase uses (S)-reticuline and O2 to produce (S)-scoulerine and H2O2.
Salutaridine synthase uses (R)-reticuline, NADPH, H+, and O2 to produce salutaridine, NADP+, and H2O. Salutaridine can then be transformed progressively to thebaine, oripavine, and morphine.
1,2-dehydroreticulinium reductase (NADPH) uses (R)-reticuline and NADP+ to produce 1,2-dehydroreticulinium, NADPH, and H+.
References
[edit]- ^ Han, Zheng; Zheng, Yunliang; Chen, Na; Luan, Lianjun; Zhou, Changxin; Gan, Lishe; Wu, Yongjiang (2008). "Simultaneous determination of four alkaloids in Lindera aggregata by ultra-high-pressure liquid chromatography–tandem mass spectrometry". Journal of Chromatography A. 1212 (1–2): 76–81. doi:10.1016/j.chroma.2008.10.017. PMID 18951552.
- ^ Dholvitayakhun, Achara; Trachoo, Nathanon; Sakee, Uthai; et al. (2013). "Potential applications for Annona squamosa leaf extract in the treatment and prevention of foodborne bacterial disease". Natural Product Communications. 8 (3): 385–388. doi:10.1177/1934578X1300800327. PMID 23678817.
- ^ a b de Morais, Liana Clébia Soares Lima; Barbosa-Filho, José Maria; de Almeida, Reinaldo Nóbrega (1998). "Central depressant effects of reticuline extracted from Ocotea duckei in rats and mice". Journal of Ethnopharmacology. 62 (1): 57–61. doi:10.1016/S0378-8741(98)00044-0. PMID 9720612.
- ^ Bradley's neurology in clinical practice. Daroff, Robert B.,, Jankovic, Joseph,, Mazziotta, John C.,, Pomeroy, Scott Loren,, Bradley, W. G. (Walter George) (Seventh ed.). London. 2015-10-25. ISBN 9780323339162. OCLC 932031625.
((cite book)): CS1 maint: location missing publisher (link) CS1 maint: others (link)
External links
[edit]![]() The dictionary definition of reticuline at Wiktionary
The dictionary definition of reticuline at Wiktionary
Opium components | |
|---|---|
| Alkaloids |
|
| Morphine group (Phenanthrenes. Includes opioids) |
|
| Isoquinolines |
|
| Protopine group |
|
| Tetrahydroprotoberberine group |
|
| Aporphine group |
|
| Phtalide-isoquinolines |
|
| α-Naphthaphenanthridines |
|
| Other components | |
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
