Phenylcopper
| |
| Names | |
|---|---|
| Other names
Copper(1+) benzenide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C 6H 5Cu | |
| Molar mass | 140.65 |
| Appearance | Crystals |
| reacts with water | |
| Related compounds | |
Related compounds
|
phenyllithium, phenylsodium, phenylcobalt |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
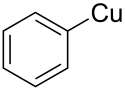
Phenylcopper is an organometallic chemical compound of copper.[1] Its chemical formula is C
6H
5Cu.[2]
Synthesis
[edit]Phenylcopper was the first known organocopper compound and was first prepared in 1923 from phenylmagnesium iodide and copper(I) iodide and in 1936 by Henry Gilman by transmetallation of phenylmagnesium iodide with copper(I) chloride.
Phenylcopper can be obtained by reacting phenyl lithium with copper(I) bromide in diethyl ether.[3]
- C6H5Li + CuBr → C6H5Cu + LiBr
Properties
[edit]Phenylcopper is a colorless solid substance that is soluble in pyridine. It can be stored for a few days without decomposition under nitrogen or in vacuum. Rapid decomposition takes place in air. Water decomposes phenylcopper to form red copper(I) oxide and varying amounts of benzene and biphenyl. It forms stable complexes with tributylphosphine and triphenylphosphine.[4]
When dissolved in dimethyl sulfide, phenylcopper forms dimers and trimers (aggregates of two or three molecules).[5]
Related structures
[edit]A diphenylcuprate(I) ion exists that can form a salt with lithium (Li+[Cu(C6H5)2]−),[5] an example of a Gilman reagent.
See also
[edit]References
[edit]- ^ Costa, G.; Camus, A.; Gatti, L.; Marsich, N. (1966-06-01). "On phenylcopper". Journal of Organometallic Chemistry. 5 (6): 568–572. doi:10.1016/S0022-328X(00)85161-X. ISSN 0022-328X. Retrieved 2 June 2021.
- ^ Frański, Rafał; Kozik, Tomasz; Staniszewski, Bartosz; Urbaniak, Włodzimierz (2010-06-01). "Phenylcopper(I) clusters in the gas phase obtained by laser desorption/ionization from bis(dibenzoylmethane)copper(II)". Open Chemistry. 8 (3): 508–512. doi:10.2478/s11532-010-0017-z. S2CID 94008194.
- ^ Ryang, Membo; Yoshida, Kunihisa; Yokoo, Hidejiro; Tsutsumi, Shigeru (April 1965). "The Reaction of Carbon Monoxide with Organometallic Compounds. X. The Reaction of Carbon Monoxide with Phenyl Derivatives of Transition Metals". Bulletin of the Chemical Society of Japan. 38 (4): 636–639. doi:10.1246/bcsj.38.636. ISSN 0009-2673.
- ^ Rappoport, Zvi; Marek, Ilan (2010). The Chemistry of Organocopper Compounds. John Wiley & Sons. p. 152. ISBN 9780470772966.
- ^ a b Bertz, Steven H.; Dabbagh, Gary; He, Xiaoming; Power, Philip P. (December 1993). "New copper chemistry. 21. Phenylcopper(I) and diphenylcuprate(I): characterization of aggregation states by carbon-13 NMR spectroscopy". Journal of the American Chemical Society. 115 (24): 11640–11641. doi:10.1021/ja00077a090.
| Cu(0,I) | |
|---|---|
| Cu(I) | |
| Cu(I,II) | |
| Cu(II) | |
| Cu(III) | |
| Cu(IV) |
|
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
