Neptunium(III) bromide
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| |||
| |||
| Properties | |||
| Br3Np | |||
| Molar mass | 477 g·mol−1 | ||
| Appearance | green solid[1] | ||
| Density | 6.62 g·cm−3[2] | ||
| Structure | |||
| α-NpBr3: hexagonal β-NpBr3: orthorhombic | |||
| α-NpBr3: P63/m (No. 176) β-NpBr3: Ccmm (No. 63) | |||
a = 791.7 pm (α), 411 pm (β), b = 791.7 pm (α), 1265 pm (β), c = 438.2 pm (α), 915 pm (β)
| |||
| Related compounds | |||
Other anions
|
neptunium(III) fluoride neptunium(III) chloride neptunium(III) iodide | ||
Other cations
|
uranium(III) bromide plutonium(III) bromide | ||
Related compounds
|
neptunium(IV) bromide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Neptunium(III) bromide is a bromide of neptunium, with the chemical formula of NpBr3.
Preparation
[edit]Neptunium(III) bromide can be prepared by reacting neptunium dioxide and aluminium bromide:[3]
- 6 NpO2 + 8 AlBr3 → 6 NpBr3 + 4 Al2O3 + 3 Br2
Properties
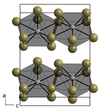
[edit]Neptunium(III) bromide is a green solid. It can crystallize in two crystal systems:
- α-NpBr3 is hexagonal with lattice parameters a = 791.7 pm and c = 438.2 pm.[2] It has the same structure as uranium trichloride.
- β-NpBr3 is orthorhombic with lattice parameters a = 411 pm, b = 1265 pm and c = 915 pm.[2] It has the same structure as the bromides from plutonium to californium.
Neptunium(III) bromide also has a green hexahydrate, which is monoclinic.[3]
Reactions
[edit]At 425 °C, neptunium(III) bromide bromide can be further brominated by bromine to form neptunium(IV) bromide.[2]
- 2 NpBr3 + Br2 → 2 NpBr4
References
[edit]- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 1969, ISBN 0-12-352651-5
- ^ a b c d Gmelins Handbuch der anorganischen Chemie, System No. 71, Transurane, Teil C, S. 148–150.
- ^ a b Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: Handbuch der Präparativen Anorganischen Chemie. 3., umgearbeitete Auflage. Band II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3, S. 1268.
External reading
[edit]- Yoshida, Zenko; Johnson, Stephen G.; Kimura, Takaumi; Krsul, John R. (2006), Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean (eds.), "Neptunium", The Chemistry of the Actinide and Transactinide Elements, Dordrecht: Springer Netherlands, pp. 699–812, doi:10.1007/1-4020-3598-5_6, ISBN 978-1-4020-3555-5, retrieved 2023-12-23
- Keller, C. (1969), "Die Chemie des Neptuniums", Anorganische Chemie, Fortschritte der Chemischen Forschung (in German), vol. 13/1, Berlin/Heidelberg: Springer-Verlag, pp. 1–124, doi:10.1007/bfb0051170, ISBN 978-3-540-04488-8, retrieved 2023-12-23
| Np(III) | |||
|---|---|---|---|
| Np(IV) |
| ||
| Np(V) | |||
| Np(VI) | |||
| Np(VII) | |||
Salts and covalent derivatives of the bromide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.