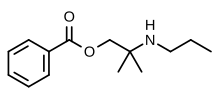
Meprylcaine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H21NO2 |
| Molar mass | 235.327 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Meprylcaine (also known as Epirocaine and Oracaine) is a local anesthetic with stimulant properties that is structurally related to dimethocaine.[1]
Meprylcaine has a relatively potent inhibitory action on the monoamine transporter and inhibits the reuptake of dopamine, norepinephrine and serotonin.[2][3]
Synthesis
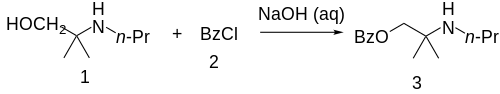
The 2-methyl-2-(propylamino)propan-1-ol [55968-10-0] (1) is treated with base and then with Benzoyl chloride (2), completing the synthesis of Meprycaine (3).
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
