Lutetium(III) chloride
| |
| Names | |
|---|---|
| IUPAC name
Lutetium(III) chloride
| |
| Other names
Lutetium chloride, lutetium trichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.205 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| LuCl3 | |
| Molar mass | 281.325 g/mol |
| Appearance | colorless or white monoclinic crystals |
| Density | 3.98 g/cm3 |
| Melting point | 905 °C (1,661 °F; 1,178 K)[3] |
| Boiling point | sublimes above 750°C[1] |
| soluble[2] | |
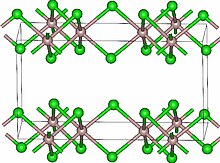
| Structure | |
| Monoclinic, mS16 | |
| C2/m, No. 12 | |
| Pharmacology | |
| License data | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling:[4][5] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Lutetium(III) oxide |
Other cations
|
Ytterbium(III) chloride Scandium(III) chloride Yttrium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals[3] and also a hygroscopic hexahydrate LuCl3·6H2O.[6] Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.[7]
Reactions
Pure lutetium metal can be produced from lutetium(III) chloride by heating it together with elemental calcium:[8]
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

