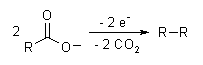Kolbe electrolysis
The Kolbe electrolysis or Kolbe reaction is an organic reaction named after Hermann Kolbe.[1] The Kolbe reaction is formally a decarboxylative dimerisation of two carboxylic acids (or carboxylate ions). The overall reaction is:
If a mixture of two different carboxylates are used, all combinations of them are generally seen as the organic product structures:
- 3 R1COO− + 3 R2COO− → R1−R1 + R1−R2 + R2−R2 + 6 CO2 + 6 e−
The reaction mechanism involves a two-stage radical process: electrochemical decarboxylation gives a radical intermediate, which combine to form a covalent bond.[2] As an example, electrolysis of acetic acid yields ethane and carbon dioxide:
- CH3COOH → CH3COO− → CH3COO· → CH3· + CO2
- 2CH3· → CH3CH3
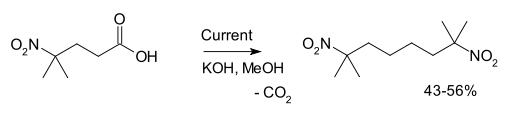
Another example is the synthesis of 2,7-dimethyl-2,7-dinitrooctane from 4-methyl-4-nitrovaleric acid:[3]
The Kolbe reaction has also been occationally used in cross-coupling reactions.
In 2022, it was discovered that the Kolbe electrolysis is enhanced if an alternating square wave current is used instead of a direct current.[4][5]
Applications
Kolbe electrolysis has a few industrial applications.[6] In one example, sebacic acid has been produced commercially by Kolbe electrolysis of adipic acid.[7]
Kolbe electrolysis has been examined for converting biomass into biodiesel[8][9] and for grafting of carbon electrodes.[10][11]
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.