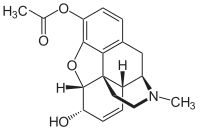
3-Monoacetylmorphine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 3-Acetylmorphine, O(3)-monoacetylmorphine |
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.208.392 |
| Chemical and physical data | |
| Formula | C19H21NO4 |
| Molar mass | 327.380 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
3-Monoacetylmorphine (3-MAM) or 3-acetylmorphine is a less active metabolite of heroin (diacetylmorphine), the other two being morphine and more active 6-monoacetylmorphine (6-MAM).
Because of the acetyl-group in 3-position, 3-MAM has relatively weak affinity to μ-opioid receptors.
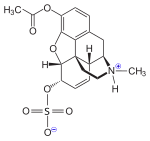
As 3-O-acetylmorphine-6-O-sulfate (C19H23NO7S), where 6-OH is changed to 6-O-SO3, it can act as a potent, centrally acting morphine derivative and has important analgesic properties.[1][2][3]

References
[edit]- ^ Houdi AA, Kottayil S, Crooks PA, Butterfield DA (March 1996). "3-O-acetylmorphine-6-O-sulfate: a potent, centrally acting morphine derivative". Pharmacology, Biochemistry, and Behavior. 53 (3): 665–671. doi:10.1016/0091-3057(95)02067-5. PMID 8866970. S2CID 14870642.
- ^ Crooks PA, Kottayil SG, Al-Ghananeem AM, Byrn SR, Butterfield DA (August 2006). "Opiate receptor binding properties of morphine-, dihydromorphine-, and codeine 6-O-sulfate ester congeners". Bioorganic & Medicinal Chemistry Letters. 16 (16): 4291–4295. doi:10.1016/j.bmcl.2006.05.060. PMID 16777416.
- ^ Brock CP, Kottayil S, Butterfield DA, Crooks PA (January 1996). "A Dihydromorphine-6-O-sulfate". Acta Crystallographica Section C. 52 (1): 122–5. doi:10.1107/S0108270195010250.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
