ئەسیتۆمێ پرێجینۆل
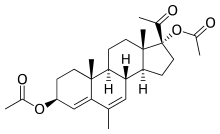
ئەسیتۆمێ پرێجینۆل، ھەروەھا وەک (مێپڕێجێنۆل دیاسێتەیت)ـش ناسراوە، لەژێر ناوی بازرگانی (دیامۆل) دەفرۆشرێ، دەرمانێکی پڕۆجستینە کە لە ڕووسیا بەکاردێت بۆ چارەسەری بۆ حاڵەتەکانی (gynecological) بەکاردیت وە ھەروەھا وەک ڕێگایەکی کۆنتڕۆلی دووگیانیش لەگەل تێکەلەی ئیسترۆجین [١][٢][٣][٤][٥][٦][٧] ھەروەھا لێکۆلینەوەی لەسەر دەکرێ بۆ چارەسەری (spontaneous abortion - لەدەستدانی دووگیانی)[٣] ھەروەھا بۆ چارەسەری ئاژەڵانیش بەکارھاتووە[٨][٩][١٠] لە ١٩٨١ـەوە خراوەتە ماڕکێتەوە بۆ فرۆشتن[٨][٩][١٠]
سەرچاوەکان
[دەستکاری]- ^ Korkhov VV (1985). "[Current trends in the development of oral contraception]". Farmakologiia I Toksikologiia (بە ڕووسی). 48 (4): 119–22. PMID 3899717.
- ^ Nikitina GV, Savchenko ON, Stepanov MG (1987). "[Hormonal properties of new 17 alpha-hydroxyprogesterone derivatives]". Problemy ĖNdokrinologii (بە ڕووسی). 33 (3): 60–3. PMID 3116530.
- ^ ئ ا Sidel'nikova VM, Demidova EM, Borisova IuF, Dondukova TM, Absava GI, Korkhov VV (1990). "[The use of acetomepegrenol in the therapy of threatened abortion]". Akusherstvo I Ginekologii︠a︡ (بە ڕووسی) (9): 37–40. PMID 2278305.
- ^ Grinenko, G. S.; Popova, E. V.; Korkhov, V. V.; Lesik, E. A.; Petrosyan, M. A.; Topil'skaya, N. I. (March 2000). "Synthesis and biological activity of 17α-acetoxy-3β-phenylpropionyloxy-6-methylpregna-4,6-dien-20-one". Pharmaceutical Chemistry Journal. 34 (3): 113–114. doi:10.1007/BF02524577. ISSN 1573-9031. S2CID 44235508.
Note that 3,17-diacetoxy-6-methylpregna-4,6-dien-20-one (1b), a structural analog of compound 1a, is certified in Russia under the trade name acetomepregnol and recommended for therapeutic purposes in gynecological practice and as a contraceptive preparation in combination with estrogens [4].
- ^ Mashkovskii, M. D. (December 2000). "Eightieth Anniversary of the Drug Chemistry Center/All-Russian Pharmaceutical Chemistry Scientific Research Institute". Pharmaceutical Chemistry Journal. 34 (12): 677–680. doi:10.1023/A:1010416205068. ISSN 1573-9031. S2CID 24703856.
- ^ Sergeev, P. V.; Rzheznikov, V. M.; Korkhov, V. V.; Grinenko, G. S.; Semeikin, A. V.; Mayatskaya, E. E.; Samoilikov, R. V.; Shimanovskii, N. L. (July 2005). "Investigation of the Gestagen Activity of 17α-acetoxy-3β-butanoyloxy-6-methylpregna-4,6-dien-20-one". Pharmaceutical Chemistry Journal. 39 (7): 358–360. doi:10.1007/s11094-005-0154-4. ISSN 1573-9031. S2CID 35450212.
Gestagens are widely used in medicine as drugs for the treatment of breast and uterine tumors, endometriosis, uterine bleeding, and premenstrual syndrome, as a means of hormonal therapy and maintenance of pregnancy, and as contraceptives [1, 2]. In clinics, drugs of this group are represented by acetomepregenol (AMP), medroxyprogesterone acetate (MPA), levonorgestrel, progesterone, didrogesterone, etc. [1].
- ^ Zeinalov, O. A.; Yaderets, V. V.; Stytsenko, T. S.; Petrosyan, M. A.; Andryushina, V. A. (July 2012). "Synthesis and biological activity of synthetic 17α-hydroxyprogesterone derivatives". Pharmaceutical Chemistry Journal. 46 (4): 203–206. doi:10.1007/s11094-012-0761-9. ISSN 1573-9031. S2CID 7159432.
- ^ ئ ا Bratanov, K., Bankov, N., Doichev, S., Pisheva, M., Klinskii, I. U., & Zhirkov, G. (1981). Action of diacetate mepregnol (diamol) on estrus induction in sheep in physiological anestrus. Reguliatsiia i intensifikatsiia protsessov razmnozheniia sel'skokhoziaistvennykh zhivotnykh: trudy Mezhdunarodnogo simpoziuma, sostoiavshegosia v Sofii, mai 1980 godina/[red. koll.: K. Bratanov (otvet. red.)... i dr.].
- ^ ئ ا Zhirkov, G. F. (1981). Testing diamol on sheep on a fattening farm. Biulleten'nauchnykh rabot-Vsesoiuznyi nauchno-issledovatel'skii institut zhivotnovodstva.
- ^ ئ ا Klinskii ID, Zhirkov GF (1982). "[Use of mepregenol diacetate (Diamol), a gestagen preparation, for estrus synchronization in caracul sheep during mating season]". Arch Exp Veterinarmed (بە ڕووسی). 36 (1): 159–62. PMID 7201304.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
